Abstract
Background: Caplacizumab (CPLZ) is a von Willebrand factor-directed antibody fragment that is indicated, in combination with therapeutic plasma exchange (TPE) and immunosuppressive therapy (IST), for the treatment of immune-mediated TTP (iTTP), also known as acquired thrombotic thrombocytopenic purpura (aTTP). While TPE is considered a mainstay of iTTP treatment, it is burdensome and associated with complications. Although reports from real-world clinical practice suggest the efficacy of TPE-free CPLZ regimens in iTTP, the use of CPLZ without first-line TPE has not been evaluated in the clinical trial setting.
Aim: To evaluate the efficacy and safety of CPLZ in combination with IST without first-line TPE in adults with iTTP.
Methods: MAYARI (NCT05468320) is a Phase 3, single-arm, open-label, multicenter study (13 countries and 49 sites) in adults experiencing an acute episode of iTTP.
Study Population: Adults with a clinical diagnosis of initial or recurrent iTTP, defined as thrombocytopenia (platelet count <100×109/L), microangiopathic hemolytic anemia, and a French thrombotic microangiopathy score of 1 or 2, are eligible for the study pending confirmation of diagnosis with an ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) activity level within 48 hours of enrollment. Exclusion criteria include other known etiologies of thrombocytopenia, serum creatinine >2.26 mg/dL if platelet count is >30×109/L, severe neurological or cardiac disease, clinically significant active bleeding or high bleeding risk, or concomitant use of chronic anticoagulant or antiplatelet therapy.
Interventions: All enrolled participants will begin CPLZ and corticosteroid therapy immediately based on the clinical diagnosis of iTTP and a French score of 1 or 2. Anti-CD20 antibody therapy can be initiated after confirmation of iTTP diagnosis (ADAMTS13 <10%). If, after initiation of CPLZ therapy, baseline ADAMTS13 activity level is found to be 10%-20%, the investigator may use their judgment to decide whether to continue CPLZ. If ADAMTS13 level is found to be >20% CPLZ should be discontinued. Otherwise, CPLZ treatment will be continued until sustained ADAMTS13 activity level of ≥50% at 2 consecutive visits after platelet count normalization. The maximum duration allowed for treatment of the presenting episode is 12 weeks (on-treatment period). The post-treatment follow-up period will be 12 weeks. Participants will not receive TPE as first-line therapy, but TPE may be started after 24 hours for a lack of adequate response per prespecified criteria or clinical deterioration at any time point.
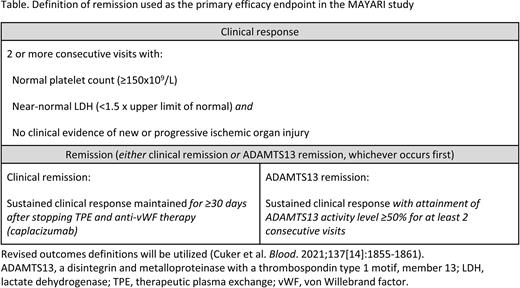
Study Outcomes: The primary endpoint is the proportion of participants achieving remission without requiring TPE during the overall study period (Table). Secondary endpoints include proportion of participants achieving remission, proportion of participants requiring TPE, proportion of participants achieving a clinical response, time to initial platelet count response (≥150×109/L sustained for ≥2 days), proportion of participants with iTTP-related exacerbation or death, refractoriness, and overall mortality. Patient-reported clinical outcome assessments will also be utilized in this study to assess impact on quality of life measures. Safety and tolerability will also be assessed. Revised outcomes definitions from the International Working Group for iTTP will be utilized (Cuker et al. Blood. 2021;137[14]:1855-1861).
Statistical Approach: An adequate number of participants will be enrolled to ensure at least 55 participants with ADAMTS13 activity levels <10% at baseline are available for analysis of the primary endpoint. It is anticipated that approximately 61 participants will be enrolled in the study. With the sample size of 55, assuming the true responder rate of participants who achieve remission without requiring TPE during the overall study period is 70%, the lower bound of 95% confidence interval would be 58%.
Summary: The current standard of care in patients with iTTP includes a combination of TPE, IST, and CPLZ. This novel study will define the efficacy and safety of CPLZ and IST without first-line TPE in adults with iTTP. This regimen would avert the risks for substantial complications associated with TPE and represents a paradigm shift in the frontline management of iTTP.
Disclosures
Gunawardena:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Hu:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Menapace:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Okada:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Accomando:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Lin:Sanofi: Current Employment, Current holder of stock options in a privately-held company.
OffLabel Disclosure:
Caplacizumab is a von Willebrand factor-directed antibody fragment that is indicated for the treatment of patients with aTTP, in combination with therapeutic plasma exchange and immunosuppressive therapy. This trial is in development to evaluate the use of caplacizumab without therapeutic plasma exchange.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal